Cannabinoids: Healing Agent for Integrative Medical Cancer Treatment.
Cannabinoids: Healing Agent for Integrative Medical Cancer Treatment:
Over the past several years, the politics and science of marijuana have been making headline news. Marijuana is categorized as a Schedule I drug by the Drug Enforcement Agency (DEA) under the Controlled Substances Act. As such, marijuana is described by the DEA as follows:
Schedule I drugs are classified as having a high potential for abuse, no currently accepted medical use in treatment in the United States, and a lack of accepted safety for use of the drug or other substance under medical supervision.1
Despite the federal government's views on marijuana, many states have pushed through legislation making marijuana available for medicinal purposes.2 These states have established laws that work to provide patients access to medical marijuana and to protect the doctors who recommend it. It should be noted that physicians do not write prescriptions for marijuana but make professional recommendations based on the patient's diagnosis and the scientific literature supporting marijuana's medicinal benefits.3
Recent changes in laws, such as the legalization of marijuana in Colorado and Washington, now allow for recreational use by decriminalizing possession of small amounts of marijuana.4 This further normalizes use and reduces the stigma and difficulty in filling medicinal cannabis prescriptions. The CNN-produced documentaries Weed and Weed2 feature Dr. Sanjay Gupta and the reversal of his position against marijuana.5 Gupta now recognizes the medical benefits of cannabis. The position of this well-known, mainstream physician and neurosurgeon echoes the shift in attitude throughout much of the US medical population and highlights the politics of pot. Physicians and patients are asking politicians and lawmakers to stand down and allow the safe prescription of medicinal cannabis and productive, impartial research to continue. Pharmaceutical companies, such as GW Pharmaceuticals in England, are advancing production, testing, and standardization. Marijuana collectives are compiling comprehensive patient outcomes from quality-of-life surveys.6,7
Understanding Cannabinoids: Going beyond THC:
The general public is for the most part familiar with tetrahydrocannabinol (THC) and its psychoactive qualities (euphoria, sedation, and appetite stimulation). Research is now blossoming around a lesser-known cannabinoid, cannabidiol, which is related to THC but does not share its psychoactive aspects.8 Cannabidiol, known by its chemical compound identifier, CBD, is present in the cannabis plant and has a wide spectrum of therapeutic abilities. Cannabidiol contains anti-inflammatory properties; has antiproliferative/anticancer effects; and can act as an antispasmodic, antimicrobial, and antipsychotic agent. Other properties include the capacity to function as a bone stimulant, a neuroprotective agent, and a vascular relaxant. Cannabidiol has also demonstrated effectiveness as a stabilizer for blood sugars, providing a potential therapy in the treatment of diabetes.9
Cannabidiol (CBD) is one of at least 60 active cannabinoids identified in the cannabis plant. It is a major constituent of the plant, accounting for up to 40% of the plant's extract, as a nonpsychotropic phytocannabinoid.10 CBD is considered to have a wider scope of medical applications than THC. An orally administered liquid containing CBD has received orphan drug status in the US, for use as a treatment for Dravet syndrome, which causes a seizure disorder in children, under the brand name, Epidiolex.11
Both THC and CBD have antinausea, neuroprotective, antianxiety, anti-inflammatory, and antiproliferative effects. These properties have been of great benefit to patients suffering from cancer and HIV.12
This once-maligned weed's medicinal effects may prove to be paradigm shifting over the next 5 to 10 years as more data are gathered and research completed. With more medical uses, an emphasis on cultivating high CBD cannabis is increasing.
Studies, Science, and Testimonials Studies:
Researchersare testing cannabinoids as a new family of antitumoral agents. Research by Arkaitz Carracedo, Meritxell Gironella, Mar Lorente, et al. is focused on pancreatic adenocarcinomas. Pancreatic adenocarcinomas are among the most malignantforms of cancer. It is of special interest to researchers to set new strategies aimed at improving the prognosis of this deadly disease. This study indicates that cannabinoid receptors are expressed in human pancreatic tumor cell lines and tumor biopsies at much higher levels than in normal pancreatic tissue.13 Studies conducted with MiaPaCa2 and Panc1 cell lines showed that cannabinoid administration (a) induced apoptosis, (b) increased ceramide levels, and (c) upregulated mRNA levels of the stress protein p8. These effects were prevented by blockade of the CB2 cannabinoid receptor or by pharmacologic inhibition of ceramide synthesis de novo. Findings indicate that cannabinoids induce apoptosis of pancreatic tumor cell lines in vitro and exert a remarkable growth-inhibiting effect in models of pancreatic cancer in vivo. The stress-regulated protein p8 is involved in THC-induced apoptosis of pancreatic tumor cells. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes.
Science:
Laboratory analysis, along with ongoing research taking place around the world, is helping us to better understand medical cannabis and the therapeutic effects of the various chemical compounds in cannabis. Understanding medicinal cannabis begins with the examination of the chemical compounds, in particular cannabinoids and terpenoids. The available chemical compounds change with how the plant is processed and administered. Potential therapeutic benefits will vary if the cannabis is processed/administered in raw (unheated), heated, or aged (degraded) form. Results also alter with the presence or absence of cannabinoid combinations. The diverse compounds in cannabis appear to modulate each other in synergistic or antagonistic ways. The California-based cannabis collective Elemental Wellness cites that the cannabinoid CBD lessens to some degree the psychotropic effects of the cannabinoid THC; however, the terpinoid a-pinene synergizes the bronchodilator effects of THC. The complexity of these chemical interactions means that medical cannabis would be best viewed as an herbal medicine, with sensitive interactions modifying the therapeutic effects as well as potential side effects. Beyond preparation and compounding considerations are the many varieties of cannabis, which includes a broad scope of variance in chemical composition. The situation is indeed challenging from the perspective of standardizing treatment.
In response to the mounting evidence and constant flow of scientific and antidotal reports, Steep Hill Halent Labs are systematically testing and publishing current information with Elemental Wellness. The collective offers educational material to its members, staff, and community physicians with the goal of educating those seeking objective input with latest scientific concepts and understanding of medical cannabis so that we may better benefit from its diverse medicinal properties. Their findings are published at www.steephillhalent.com/resources.
Testimonial Accounts:
The highly publicized video Run from the Cure documents a cancer sufferer in Canada, Rick Simpson, who used an extract of cannabis to successfully treat his mesothelioma and who created Phoenix Tears, a high-potency cannabis oil treatment that many patients are taking into their own hands to manufacture and administer as a last resort for terminal cancer and other life-threatening diseases.14,15
Patients have been known to use raw cannabis as an ingredient in juices and smoothies. Unlike cooked cannabis products, raw cannabis does not appear to activate the THC compound, allowing for medicinal properties to be isolated without any psychoactive effects. There is an emerging groundswell of patient self-care filling the gap between doctors' ability to prescribe, lagging legal formulas, politics, and patients' needs.
Cannabidiol can be used along with traditional cancer care in the treatment of side effects brought on by chemotherapy and radiation. Both THC and CBD have antinausea, neuroprotective, antianxiety, anti-inflammatory, and antiproliferative effects.16 These characteristics may play an important role in helping patients endure some of the hardships faced during treatment.
Cannabis is now being referred to as a "pharmaceutical treasure trove." Active practitioners are using science to inform and instruct for the optimal utilization. Efficient and effective treatment is the underlying motivation in bringing this ancient herbal medicine into treatment modalities that reduce suffering, indicate curative properties, and can be safely combined with other therapies.
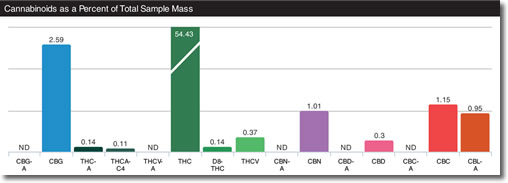
Partial List of Commonly Recognized Cannabinoids and Their Chemical Element Abbreviations17:
CBGA Cannabigerolic acid
CBGVA Cannabigerivarinic acid
CBG Cannabigerol
CBGV Cannabigerivarin
THCA Tetrahydrocannabinolic acid
THCVA Tetrahydrocannabivarinic acid
THC Tetrahydrocannabivarin
THCV Cannabinolic acid
CBN Cannabinol
CBDA Cannabidiolic acid
CBDVA Cannabidivaric acid
CBD Cannabidiol
CBDV Cannabidivarin
CBCA Cannabichromic acid
CBCA Cannabichromivaric acid
CBC Cannabichromene
CBCV Cannabichromivarin
CBLA Cannabicyclol acid
CBL Cannabicyclol
Cannabinoids in the Cancer Patient The specific applications for the cannabinoids in the cancer patient may include18:
1. antiproliferation
2. antiemetic
3. neuroprotection (i.e., for patients on platin-containing drugs)
4. anti-inflammation
5. analgesic
6. bone stimulation
Six cannabis compounds have been documented to act as antiproliferative agents, reducing cancer cell growth: THCA, CBDA, CBD, CBC, CBG, and THC.19
Medical marijuana testing and use as a nausea suppressant and appetite stimulant has been around since the 1970s. As early as 2003, GW Pharmaceuticals and Bayer AG were looking toward a safer alternative to smoking cannabis and with a mechanism that allowed for the quantifiable administration of cannabinoids.20 They developed a medicinal cannabis extract known as Sativex, which contains THC and CBD; it is administered by spraying it into the mouth. This drug is now legal in Canada under the name Nabiximols (USAN, trade name Sativex). This aerosolized mist for oral administration containing a near 1:1 ratio of CBD and THC. In 2005 Nabiximols was approved by Canadian authorities to alleviate pain associated with multiple sclerosis.21
Major laboratories are deeply involved in the pharmacokinetics of cannabis, yet in the US the results and formulas are often not readily available to practitioners today.22 As the medical market develops, and more growers demonstrate their capacities to produce and reproduce cannabis strains with consistent cannabinoid profiles, a registry and further quantification of cannabinoids may become more accessible for treatment.23
Here are some of the pharmaceutical cancer treatment applications that contain cannabinoids or their extracts approved or in the approval process today.
Drugs That Contain Chemicals Taken Directly from the Marijuana Plant24
Sativex
Manufacturer: GW Pharmaceuticals (GWPH on NASDAQ)
Sativex oral spray
Source: "Medical Marijuana aka Sativex Now Available in UK." examiner.com. June 19, 2010.
Cannabis-related properties:Mouth spray whose chemical compound is derived from natural extracts of the cannabis plant. Sativex contains two cannabinoids: THC (delta-9-tetrahydrocannabinol) and CBD (cannabidiol).
Suggested medical use: Treatment of neuropathic pain and spasticity in patients with multiple sclerosis (MS); analgesic treatment in adult patients with advanced cancer who experience moderate to severe pain.
Approved and launched in the UK on June 21, 2010, making it the first cannabis-based prescription medicine in the world (rescheduled from UK Schedule 1 to Schedule 4 on Apr. 10, 2013). Licensed to Bayer in the UK and to Almirall in Europe. Approved to treat spasticity caused by multiple sclerosis in Spain (July 28, 2010), Canada (August 31, 2010), Czech Republic (April 15, 2011), Denmark (June 8, 2011), Germany (July 4, 2011), Sweden (December 22, 2011), Austria (February 7, 2012), Italy (May 7, 2013), and Switzerland (November 27, 2013). Also approved in Finland, Israel, Norway, and Poland.
In the US, phase III clinical trials started in late 2006 for treatment of pain in cancer patients and were in recruitment in 2013. On April 20, 2011, a US patent was granted for Sativex in cancer pain. As of April 28, 2014, Sativex was still in phase III clinical development to treat pain in cancer patients, and the company expects to see results from the program at the end of 2014. On April 28, 2014, the FDA granted "Fast Track" designation to Sativex for the treatment of pain in patients with advanced cancer. The FDA website says, "Fast track is a process designed to facilitate the development, and expedite the review of drugs to treat serious conditions and fill an unmet medical need."
GW Pharmaceuticals worked with US licensing partner Otsuka Pharmaceutical to open a Phase III Investigational New Drug application in the US to treat spasticity due to multiple sclerosis on August 14, 2013. The US phase III trial was expected to begin in the second half of 2014.
Drugs That Contain Synthetic Versions of Chemicals Naturally Found in Marijuana
Dronabinol/Marinol
Manufacturer: Unimed Pharmaceuticals, a subsidiary of Solvay Pharmaceuticals
Marinol:Source: "Cannabis, Coca, & Poppy: Nature's Addictive Plants," deamuseum.org (accessed Nov. 12, 2013).
Cannabis-related properties:Synthetic Delta-9 THC.
Suggested medical use:Treatment of nausea and vomiting for patients in cancer treatment; appetite stimulant for AIDS patients; analgesic to ease neuropathic pain in multiple sclerosis patients.
Approval Status:
FDA approved in US as Schedule I drug for appetite stimulation (1992) and for nausea (1985); moved to Schedule III effective July 2, 1999.
Approved in Denmark for multiple sclerosis (Sep. 2003).
Approved in Canada for AIDS-related anorexia (Apr. 2000) and for nausea and vomiting associated with cancer chemotherapy (1988).
Drugs That Contain Chemicals Similar to Those in Marijuana but Not Found in the Plant
Nabilone/Cesamet
Manufacturer: Valeant Pharmaceuticals International (VRX on NASDAQ)
Cannabis-related properties:Synthetic cannabinoid similar to THC.
Suggested medical use:Treatment of nausea and vomiting in patients undergoing cancer treatment.
Approval Status:
Originally approved by the FDA for use in the US in 1985, but removed from the market until reapproved by the FDA on May 15, 2006, and made available in US pharmacies on August 17, 2006. Also approved in UK and Australia (1982), Canada (1981), and Mexico (2007).
On May 15, 2006, the FDA approved safety labeling revisions for nabilone (Cesamet 1-mg capsules) to advise of warnings and precautions related to its use, such as its potential to affect the mental state of a patient. On February 22, 2007, Valeant announced the submission of an Investigational New Drug application to test Cesamet as a treatment for chemotherapy-induced neuropathic pain.
Dexanabinol:
Manufacturer: Solvay Pharmaceuticals (acquired by Abbott Laboratories in 2010; ABT on NASDAQ)
Cannabis-related properties:Synthetic nonpsychotropic cannabinoid that blocks NMDA receptors and COX-2 cytokines and chemokines.
Suggested medical use: Neuroprotective (protects brain from damage) for use after cardiac surgery; regain memory and other high-level function following traumatic brain injury (TBI); possible future use as an anticancer drug.
Approval Status:
Not approved for use as of November 11, 2013.
The phase III clinical trial involving 846 patients was completed in December 2004; Pharmos said that the drug failed to show statistically significant improvement in the late-stage clinical trial; a phase I study to test for the treatment of brain cancer began in September 2012.
A Note on Potential Integrative Therapies:
When dealing with patients with cancer or any life-threatening diagnosis, we must always work with our colleagues in traditional and conventional medicine. When treating cancer patients it is strongly advised that they all have a fellowship-trained oncologist on their medical team.
All patients need to be advised through written consent that any integrative, complementary, or alternative approaches that they chose to pursue may not be the standard of care as recognized by the AMA or the FDA. All patients should be advised of all the traditional treatments available to them and be referred to the appropriate specialists as needed and requested.
Transparency of care is extremely important when practicing integrative or functional oncology. Documentation must be detailed and should include rationale for care and reasoning for implementing integrative therapies (e.g., patient's refusal of traditional care). All health-care practitioners are encouraged work openly and collaborate with the whole care team.
Part of an Integrated Treatment Plan:
Medicinal use of cannabis is not usually a stand-alone treatment plan. In my experience it can be successfully utilized as part of a comprehensive care program for any patient who may benefit from its wide range of attributes.
We are looking toward advanced clinical trials for palliative cancer care and bringing these into an integrative approach that includes both prevention and treatment. Nausea, vomiting, and appetite suppression are serious, potentially life-threatening side effects of chemotherapy. These effects can be so detrimental that patients may choose to discontinue therapy rather than face the rigors of the side effects. THC has been tested and is effective in nausea prevention, pain inhibition, and appetite stimulation.25 THC, taken as a viable, encapsulated formula or tincture, or through smoke/vapor inhalation, has tested and is effective as an analgesic, antidepressant, and sedative and for the muscle spasms that can be a debilitating symptom of multiple sclerosis.26
Pharmacology Reduction:
Many of the patients whom I routinely see are on a wide range of prescription medications. These drugs have many side effects, are expensive, and often compromise wellness in a number of ways. Physicians tread a delicate path, balancing side effects, complications, and adverse drug interactions. I encourage patients to examine places where their quality of life can be improved by reducing prescription drugs. Overall, by reducing the hazards of polypharmacy, some harm reduction can be achieved.27
Potential Side Effects and Minimizing Them:
Cannabinoids have an overall stable and sound drug safety profile. There are no substantiated acute fatal cases due to cannabis use in humans.28 Cannabinoids are usually well tolerated, both in treatment and study groups for humans and animals. Beyond this, cannabinoids do not produce the generalized toxic effects of most conventional chemotherapeutic agents. According to a study conducted by Manuel Guzmán, THC treatment tended to increase survival and lower the incidence of primary tumors. "Similarly, long-term epidemiological surveys, although scarce and difficult to design and interpret, usually show that neither patients under prolonged medical cannabinoid treatment nor regular cannabis smokers have marked alterations in a wide array of physiological, neurological and blood tests. The use of cannabinoids in medicine, however, is severely limited by their psychoactive effects."29
This underscores the significance of CBD based formulas, which reduce the THC and psychoactive qualities and expand potential applications for cannabinoid-based treatment options.
Conclusion:
This is a case in which science and society are coming together to drive change. It is only a matter of time before cannabinoid pharmacology research and development will create a structured system for exacting cannabinoid dosage and delivery methods.
Expect measured, quantified dosages of pharmaceutical formulas containing marijuana chemical extractions within the decade. Both the pharmaceutical and tobacco industries have been eyeing medicinal and recreational marijuana for quite some time. As the political and social climate around marijuana evolves, we should expect to see both of these groups start to participate more in the development and manufacture of marijuana-based products. Meanwhile, there is ample opportunity to contribute to research and witness a new course of medicines being refined with testing analysis being compiled and released for public consumption.
The number of firsthand testimonials for cancer treatment resulting in remission, supporting chemotherapy and pain and symptom management is mounting. These accounts mesh with scientific data to make a powerful case for future research and clinical applications.
References:
Steep Hill Halent Labs has conducted comprehensive research. See published reports and the 14-page document that it produced in partnership with Elemental Wellness, Understanding Medical Cannabis, at steephilllab.com/resources.
Additional information can be found at www.icurecancer.com
Notes:
1. Drug Enforcement Administration. Drug fact sheet: marijuana [online document]. www.justice.gov/dea/druginfo/drug_data_sheets/Marijuana.pdf
2. 21 legal medical marijuana states and DC [Web page]. ProCon.org. medicalmarijuana.procon.org/view.resource.php?resourceID=000881.
3. Kuen BM. JAMA scopes out Colorado [online article]. O'Shaughnessy's online. May 14, 2014. www.beyondthc.com/jama-scopes-out-colorado
4. State marijuana laws map [Web page]. Governing.com. April 14, 2014. www.governing.com/gov-data/state-marijuana-laws-map-medical-recreational.html.
5. Gupta S. Weed 2. CNN. March 11, 2014. Trailer at www.cnn.com/SPECIALS/health/medical-marijuana
6. GW Pharmaceuticals. Cannabinoids and cannabis-based medicines [Web page]. www.gwpharm.com/faqs.aspx
7. Understanding medical cannabis [online document]. Elemental Wellness Center. August 2013. elementalwellnesscenter.com/UnderstandingCannabis.pdf.
8. CBD rich medicine [Web page]. Harborside Health Center. www.harborsidehealthcenter.com/CBD-rich-medicine.html
9. Frisher M, White S, Varbiro G, et al. The role of cannabis and cannabinoids in diabetes. Br J Diabetes Vasc Dis. 2010;10(6)267-273. Available at www.medscape.com/viewarticle/738863_3.
10. Campos AC, Moreira, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3364-3678. www.ncbi.nlm.nih.gov/pmc/articles/PMC3481531.
11. Maa E, Gedde M. Next generation sequencing and Dravet's syndrome [Web page]. www.medicinalgenomics.com/dravets
12. Sancho R, de la Vega L, Macho A, Appendino G, Marzo VD, Muñoz E. Mechanisms of HIV-1 inhibition by the lipid mediator N-arachidonoyldopamine. J Immunol. 2005;175:3990-3999. Available at mycbdresearch.com/mechanisms-of-hiv-1-inhibition-by-the-lipid-mediator-narachidonoyldopamine.
13. Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar M. Cancer treatment: progress and promise. Cancer Res. January 15, 2008;68;339 Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3364-78.. Available at cancerres.aacrjournals.org/content/68/2/339.full?sid=3491ce05-d9ce-488b-8da1-abdca15ccc3c.
14. Simpson R. Run from the Cure. Film. December 6, 2009. Available at https://www.youtube.com/watch?v=0psJhQHk_GI
15. Boise E, Boise B. Our journey with 4 stage cancer, cannabis and alternative therapies [website]. April 2014. cannabis-cancer-info.org
16. Cohen J. Cannabidiol or CBD [Web page]. Holos Health. www.holoshealth.org/Cannabidiol__CBD_.html.
17. Understanding medical cannabis. Op cit.
18. Ibid.
19. Guzmán M. Cannabinoids: potential anticancer agents. Nat Rev Cancer. October 2003;3:745-755. Available at www.nature.com/nrc/journal/v3/n10/full/nrc1188.html
20. Cannabis-based medicines-GW pharmaceuticals: high CBD, high THC, medicinal cannabis. Drugs R D. 2003;4(5):306-309. www.ncbi.nlm.nih.gov/pubmed/12952500
21. GW receives qualifying notice for approval in canada for Sativex [online press release]. October 22, 2007: www.gwpharm.com/GW%20receives %20Qualifying%20Notice%20for%20approval
%20in%20Canada%20for%20Sativex.aspx
22. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. Aug 2007;4(8):1770-1804. www.ncbi.nlm.nih.gov/pmc/articles/PMC2689518
23. Sexton M, Ziskind J. Sampling cannabis for analytical purposes[online document]. Botec Analysis Corp. November 15, 2013. liq.wa.gov/publications/Marijuana/BOTEC%20reports/1e-Sampling-Lots-Final.pdf.
24. 10 pharmaceutical drugs based on cannabis [Web page]. ProCon.org. 2014. medicalmarijuana.procon.org/view.resource.php?resourceID=000883.
25. Questions and answers about cannabis [Web page]. National Cancer Institute. March 25, 2014 www.cancer.gov/cancertopics/pdq/cam/cannabis/patient/page2
26. Multiple Sclerosis [Web page]. NORML. norml.org/library/item/multiple-sclerosis.
27. Woodruff K. Preventing polypharmacy in older adults. Am Nurse Today. October 2010;5(10). Available at www.americannursetoday.com/article.aspx?id=7132&fid=6852
28. Russo EB. Cannabis and Cannabinoids: Pharmacology, Toxicology, and Therapeutic Potential. September 5, 2013.Available at books.google.com/books/about/Cannabis_and_Cannabinoids.html?id=XfW3AAAAQBAJ.
29. Guzmán. Op cit.

Sean Devlin, DO, HMD, is a board-certified family physician and board eligible in emergency medicine. Dr. Devlin is board certified and fellowship trained in anti-aging and regenerative medicine and fellowship trained in integrative cancer therapeutics. He holds a master's degree in biochemistry and has pursued doctoral studies in pharmacology with an emphasis on the evaluation of novel antineoplastic agents. Dr. Devlin has been practicing integrative oncology for the past 10 years and is a certified instructor of IPTLD and sits on the advisory board of the IPTLD foundation.
He has traveled extensively working with cancer physicians and researchers internationally in an effort to better understand cancer and its treatment. Dr. Devlin currently teaches for and works with the AAAAM Integrative Cancer Therapeutics fellowship and master's-degree program through the University of South Florida. He is a frequent speaker and guest lecturer throughout the US and currently serves on the medical advisory board for the Best Answer For Cancer Foundation.
Dr. Devlin and his team also provide onsite training services for physicians, medical staff, and front office staff, and he works with a variety of integrative-oncology and specialty medicine groups around the country as a consultant.
He has been fellowship trained in neuromuscular medicine and has spent many years working with patients suffering from chronic pain conditions. He has focused on rehabilitating patients and getting patients transitioned off of narcotics and other controlled substances.Dr. Devlin is licensed in Nevada, California, and Colorado. He is the medical director of Highland Springs Wellness Center in Grass Valley, California, and is a consultant at Sierra Integrative Medical Center, Reno, Nevada.
Bron: www.thehealthcure.org

